The panel discusses the evolving treatment landscape for mCRPC, focusing on the role of RLTs and PARP inhibitors.
Prostate Cancer
Advertisement
Latest News
The panel discusses treatment intensification strategies for mCSPC, emphasizing the shift toward doublet and triplet therapy.
The panel discusses the growing role of advanced molecular imaging and genomic testing in prostate cancer care.
The panel begins with the landscape of advanced PC care, emphasizing accurate categorization of disease stage and burden.
Drs. Ahmed and Ciuro discuss the real-world quality-of-life improvements patients may experience after de-escalating therapy.
Drs. Ahmed and Ciuro explore the rationale and complexity behind treatment de-escalation in de novo oligometastatic HSPC.
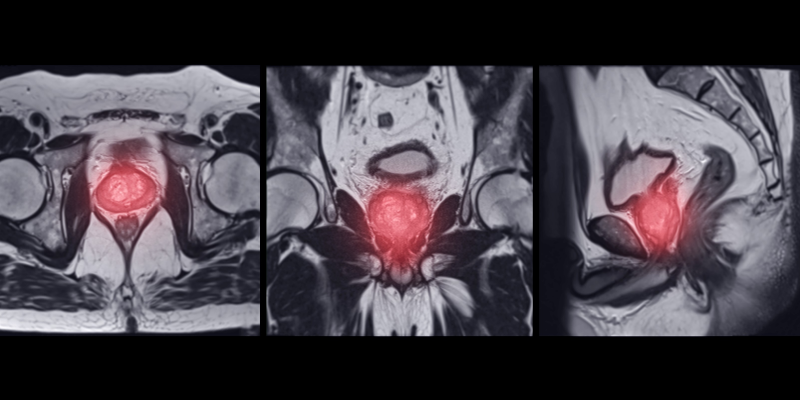
Dr. Kimura shares insights on PSMA PET/CT metrics and their potential role in guiding treatment decisions for mCRPC.
Prostate Cancer Knowledge Hubs
Curated clinical content on prostate cancer types, therapies, and technologies
Conference Coverage
Dr. Kimura shares insights on PSMA PET/CT metrics and their potential role in guiding treatment decisions for mCRPC.
Professor Bex discusses the phase II NEOAVAX trial that evaluated avelumab/axitinib as neoadjuvant therapy in localized RCC.
Samer Srour, MB, talks about the updated ALLO-316 and its use in targeting CD70 in patients with advanced ccRCC.
Dr. Flavell discusses how landmark studies have shaped current trial designs, and growing interest in combination therapies.
Dr. Flavell highlights the expansion of lutetium PSMA therapies into earlier disease settings: HSPC and oligometastatic PC.
Preliminary trial data suggests that 18F-DCFPyL PSMA PET/CT combined with mpMRI may improve csPCA detection over MRI alone.












 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.