Almost 30% of patients will experience recurrence while undergoing EBRT that will require ADT and salvage therapies.
Localized Prostate Cancer
Advertisement
Latest News
Dr. James showcases an MMAI model to identify benefit from 2nd-generation ARPIs in hr/nm PCa patients from STAMPEDE.
A novel algorithm identifies patients with high-risk non-metastatic PCa who may benefit from second-generation ARPIs.
Dr. Evan Kovac speaks about his pioneering work with extended reality display technology in urologic surgery and education.
The use of MRI-based monitoring can aid in the avoidance of repeat biopsies, and reduce the chances of undergoing surgery.
Dr. Avudaiappan compares radical prostatectomy with dose-escalated radiation with ADT for the treatment of prostate cancer.
Drs. Posadas and Ambinder reflect on the first AUA guideline update for salvage therapy in prostate cancer in over a decade.
Prostate Cancer Knowledge Hubs
Curated clinical content based on prostate cancer types, therapies, and technologies
Conference Coverage
Dr. Kimura shares insights on PSMA PET/CT metrics and their potential role in guiding treatment decisions for mCRPC.
Professor Bex discusses the phase II NEOAVAX trial that evaluated avelumab/axitinib as neoadjuvant therapy in localized RCC.
Samer Srour, MB, talks about the updated ALLO-316 and its use in targeting CD70 in patients with advanced ccRCC.
Dr. Flavell discusses how landmark studies have shaped current trial designs, and growing interest in combination therapies.
Dr. Flavell highlights the expansion of lutetium PSMA therapies into earlier disease settings: HSPC and oligometastatic PC.
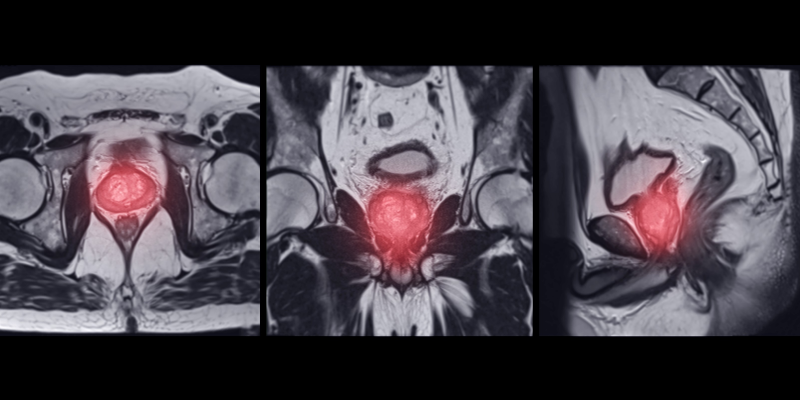
Preliminary trial data suggests that 18F-DCFPyL PSMA PET/CT combined with mpMRI may improve csPCA detection over MRI alone.











 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.