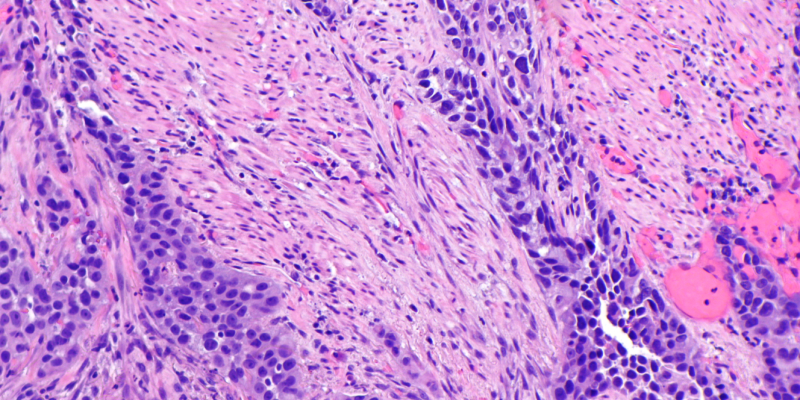
Researchers find promise for ctDNA-guided immunotherapy for patients with MIBC who underwent radical cystectomy.
Muscle Invasive Urothelial Carcinoma
Advertisement
Latest News
[68Ga]-FAPI-PET/MRI and [18F]-FDG-PET/CT share similar diagnostic accuracy for tumor staging in patients with MIBC.
At the time of a pre-specified interim analysis, significant improvements in EFS and OS were seen.
Dr. Plimack details RETAIN, from early findings with neoadjuvant ddMVAC to the evolution of RETAIN-1 and RETAIN-2.
Preliminary results of the study showed promising efficacy and safety with neoadjuvant treatment with DV plus toripalimab.
Drs. Ghatalia and Nizam break down in detail the RETAIN-2 study, a phase 2 trial of risk enabled therapy for MIBC.
Dr. Galsky highlights the latest findings on the impact of pCR on long-term outcomes in the NIAGARA trial.
Urothelial Carcinoma Knowledge Hubs
Curated clinical content based on urothelial cancer types, therapies, and technologies
Conference Coverage
Dr. Canes discusses the formation of the WellPrept delivery platform and how it aims to boost patient education.
Mohummad Siddiqui, MD, discusses his work with the Commission on Cancer and their development on standards of care.
John P. Sfakianos, MD, discusses the history and use of ctDNA in the GU oncology field.
Dr. Sandoval chats with David Ambinder, MD, on the upstaging of cT1b and cT2 tumors to pT3a.
Maximizing Benefits and Minimizing Harm: Reviewing AUA Guidelines on Prostate Cancer Early Detection
Dr. Salami explains how updated guideline approaches shared decision-making in higher-risk groups.Dr. Cheaib discusses outcomes in patients with non-clear cell RCC with tumor thrombus involvement after surgical treatment.










 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.