Renal Cell Carcinoma
Advertisement
Drs. Karine Tawagi and Samantha Armstrong share their thoughts on KEYNOTE-564, NEOAVAX, and more.
Drs. Wulff-Burchfield and Beckermann offer perspective on how patient-driven science is shaping QOL measurement.
Drs. Wulff-Burchfield and Beckermann share how they help GU patients make informed, personalized treatment decisions.
Drs. Wulff-Burchfield and Beckermann discuss how GU oncologists can build trust and deepen patient connections.
Machine learning may be a useful tool in predicting renal cell carcinoma tumor response to nivolumab monotherapy.
Dr. Canes discusses the formation of the WellPrept delivery platform and how it aims to boost patient education.
Mohummad Siddiqui, MD, discusses his work with the Commission on Cancer and their development on standards of care.
John P. Sfakianos, MD, discusses the history and use of ctDNA in the GU oncology field.
Dr. Sandoval chats with David Ambinder, MD, on the upstaging of cT1b and cT2 tumors to pT3a.
Dr. Cheaib discusses outcomes in patients with non-clear cell RCC with tumor thrombus involvement after surgical treatment.
Drs. Ben-David and Joyce evaluate the performance of ctDNA in detecting localized RCC disease recurrence after surgery.
Drs. Ben-David, Joyce share an analysis on recurrence-free survival according to ctDNA status in patients with renal masses.
Patients with mRCC and primary resistant disease to nivolumab plus ipilimumab have worse survival outcomes.
CN may improve OS for patients with metastatic nccRCC compared with systemic therapy alone, especially when combined with IO.
A research analysis evaluated tumor-informed ctDNA analysis versus traditional imaging techniques for localized RCC.
Pre-op ctDNA in localized RCC is linked to aggressive tumor features and worse RFS, highlighting its prognostic potential.
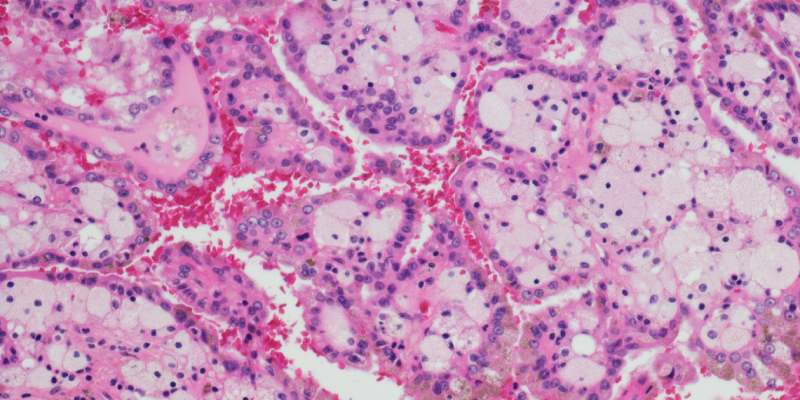
Histologic subtype did not significantly affect 5-year cancer-specific survival after robotic partial nephrectomy for RCC.
Patients with ccRCC and nccRCC with tumor thrombus have similar survival outcomes after surgical resection.
Dr. Brugarolas reflects on his lab's role in developing HIF-2 inhibitors and his ongoing work to improve patient outcomes.
An exploratory analysis revealed a strong OS advantage associated with a PD-L1 combined positive score higher than 1.













 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.