Non-Muscle Invasive Urothelial Carcinoma
Advertisement
Researchers find promise in Gem/Doce as an alternative therapy for patients with NMIBC recurrence after BCG induction.
Drs. Packiam and Yerram conclude with an AI-powered pathology tool designed to predict response to BCG in NMIBC.
Drs. Packiam and Yerram shift focus to SunRISe-1 Cohort 4, which examined BCG-unresponsive papillary disease.
Drs. Packiam and Yerram contextualize recent data from the CREST trial, which evaluated sasanlimab with BCG for HR NMIBC.
Drs. Packiam and Yerram focus on how agents like gem/doce, pembro, nadofaragene, cretostimogene, and TAR-200 are used.
Drs. Packiam and Yerram discuss TAR-200 and the ongoing SunRISe-3 trial exploring its effectiveness amid BCG shortages.
Drs. Packiam and Yerram discuss the emergence of gem/doce as a promising alternative to BCG.
The study’s major efficacy outcomes included a complete response in 78% of patients.
Dr. Hayne speaks about the ANZUP 101 trial results that evaluated the combination of mitomycin and BCG for NMIBC.
BCG plus mitomycin may be an alternative treatment for patients with high-risk NMBIC as access to BCG is limited worldwide.
Drs. Galsky and Tawagi detail the event-free survival subgroup analyses based on disease stage from the CREST study.
Updated CREST trial explores sasanlimab plus BCG in BCG-naive, high-risk NMIBC, with new insights by disease stage and tumor.
Dr. Brown breaks down the CREST trial results in high-risk NMIBC, highlighting the benefits of combining sasanlimab with BCG.
Adding one year of treatment with durvalumab to BCG can extend the time that patients live without disease recurrence.
Dr. Gomella and Ambinder provide a comprehensive overview of the growing and changing treatment landscape for NMIBC.
Dr. Tyson discusses real-world decision-making around bladder-sparing options for BCG-unresponsive NMIBC.
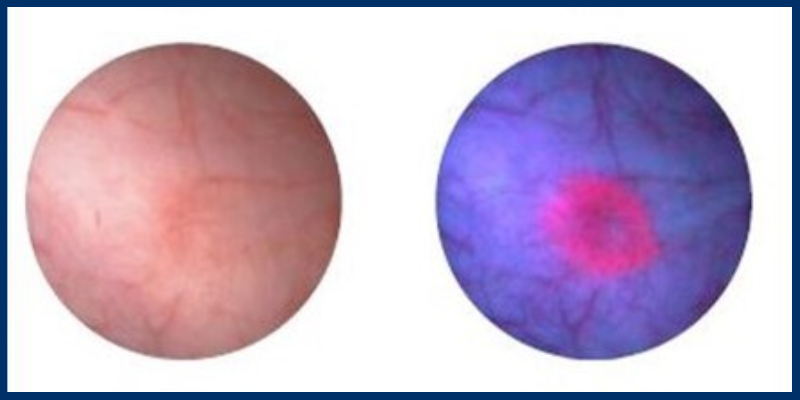
Previous research has shown that BLC can provide improved detection rates, mainly for high-risk disease and invasive tumors.
Noah Hahn, MD, gives an overview of durvalumab with intravesical gemcitabine and docetaxel for BCG-unresponsive NMIBC.
This new research shows no link between patient BMI and NMIBC recurrence, stage progression, or grade progression.
Sasanlimab is being further studied in combination with other antibody drug conjugate treatments in advanced solid tumors.













 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.